The journey of rapamycin from a soil-derived antifungal compound discovered on Easter Island to one of the most studied longevity interventions represents a remarkable evolution in pharmaceutical science. Initially developed as an immunosuppressant for organ transplantation, rapamycin has emerged as the leading candidate for pharmacological intervention in aging, supported by unprecedented consistency across species and growing human clinical evidence. This comprehensive analysis examines the current state of rapamycin research, its transition from immunosuppression to longevity medicine, and the implications for human healthspan extension.

Historical Development and FDA Approval
Rapamycin's discovery story begins in the 1960s when researchers isolated the compound from Streptomyces hygroscopicus bacteria found in soil samples from Easter Island (Rapa Nui). Initially investigated for its antifungal properties, the compound's immunosuppressive capabilities soon became apparent, leading to its development as a transplant rejection prevention medication. The FDA approved rapamycin (marketed as Rapamune) in 1999 specifically for preventing kidney transplant rejection, marking the beginning of its clinical application.
The drug's mechanism of action centers on its inhibition of the mechanistic Target of Rapamycin (mTOR) pathway, a discovery that would later prove crucial to understanding its anti-aging properties. The mTOR pathway serves as a central regulator of cellular metabolism, growth, and survival, integrating signals from nutrients, growth factors, and cellular energy status. This mechanism initially made rapamycin valuable as an immunosuppressant but would later position it as a potential intervention for aging-related processes.
Beyond transplantation medicine, rapamycin found applications in oncology and rare genetic disorders. It received approval for treating certain cancers, including renal cell carcinoma and mantle cell lymphoma, and demonstrated efficacy in managing tuberous sclerosis complex, a genetic disorder characterized by benign tumor growth. These diverse therapeutic applications foreshadowed the drug's potential broader impact on cellular aging processes.
The mTOR Pathway and Aging Mechanisms
The mechanistic Target of Rapamycin (mTOR) pathway represents one of the most extensively studied longevity-regulating networks in biology. As a central nutrient-sensing pathway, mTOR integrates environmental cues including amino acids, glucose, growth factors, and cellular energy status to coordinate fundamental cellular processes. The pathway exists as two distinct complexes: mTORC1 (mTOR Complex 1) and mTORC2 (mTOR Complex 2), each with different functions and sensitivity to rapamycin.
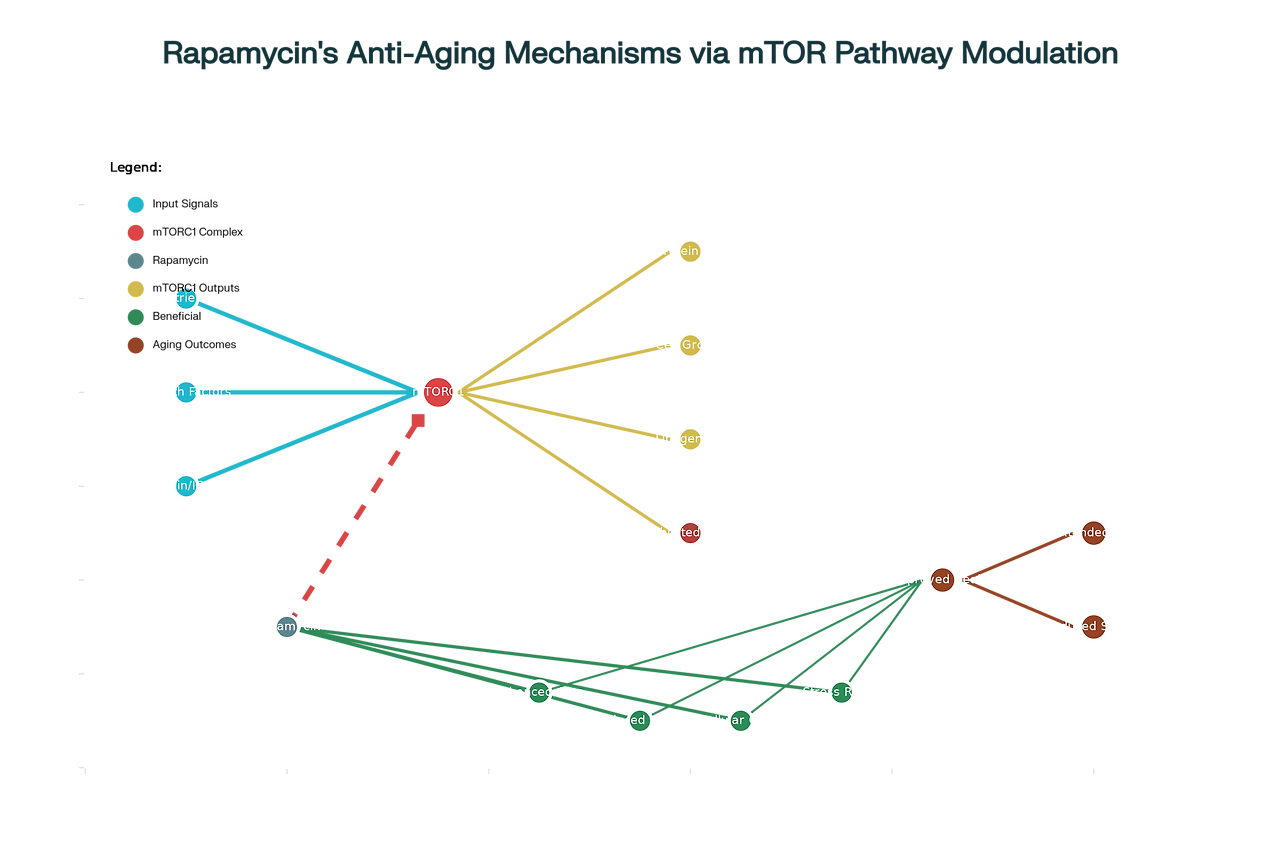
mTORC1 activation promotes anabolic processes including protein synthesis, lipogenesis, and nucleotide synthesis while simultaneously inhibiting catabolic processes such as autophagy. Under conditions of nutrient abundance and growth factor stimulation, elevated mTORC1 activity drives cellular growth and proliferation. However, chronic hyperactivation of this pathway has been implicated in accelerated aging and age-related disease development. Conversely, mTORC1 inhibition shifts cellular resources toward maintenance and repair processes, including enhanced autophagy, stress resistance, and cellular quality control mechanisms.
The aging connection emerged from observations that mTOR signaling increases with age in various tissues, contributing to the accumulation of cellular damage, metabolic dysfunction, and chronic inflammation characteristic of aging. Rapamycin's selective inhibition of mTORC1 effectively reverses many of these age-related changes. The drug enhances autophagy, the cellular process responsible for removing damaged organelles and misfolded proteins. This enhanced cellular cleanup reduces the accumulation of senescent cells and their harmful secretory products, known as the senescence-associated secretory phenotype (SASP).
Furthermore, rapamycin modulates immune function in complex ways that may benefit aging individuals. While it suppresses certain immune responses, low-dose intermittent administration has been shown to enhance immune function in elderly populations, potentially reversing immunosenescence—the age-related decline in immune system effectiveness. Studies have demonstrated that rapamycin can improve vaccine responses and reduce inflammatory markers associated with aging.
Animal Studies and Lifespan Extension Evidence
The evidence for rapamycin's life-extending effects across multiple species is unprecedented in aging research. Beginning with studies in yeast, worms, and flies, rapamycin consistently demonstrated the ability to extend lifespan and improve healthspan measures across diverse model organisms. However, the landmark studies that truly established rapamycin's potential were conducted in mice through the National Institute on Aging's Interventions Testing Program (ITP).
The original ITP studies, initiated in 2004, demonstrated that rapamycin could extend both median and maximum lifespan in genetically heterogeneous mice when treatment began at 20 months of age—equivalent to about 60 years in humans. Male mice showed a 9% increase in median lifespan, while females experienced a 14% increase. Remarkably, even when treatment began later in life, rapamycin maintained its life-extending effects, suggesting that the intervention could benefit individuals who start treatment in middle age or beyond.
A comprehensive meta-analysis published in 2025 examined data from 167 studies across eight vertebrate species, including zebrafish, mice, rats, and rhesus macaques. This analysis confirmed that rapamycin extends lifespan as consistently as dietary restriction—long considered the gold standard for longevity interventions—across all studied vertebrate species. The study found no sex-specific differences in rapamycin's effects, suggesting that both males and females benefit equally from treatment.
Recent breakthrough research has demonstrated that combining rapamycin with other compounds can produce additive longevity effects. A 2025 study published in Nature Aging showed that combining rapamycin with trametinib, an FDA-approved cancer drug that inhibits the MEK pathway, extended mouse lifespan by 27-30%—significantly greater than either drug alone. This combination therapy also reduced tumor development, decreased inflammation, and improved metabolic health markers.
Human Clinical Trials and Emerging Evidence
The translation of rapamycin's promising animal results to human studies has accelerated dramatically in recent years, with multiple clinical trials investigating its effects on aging and age-related conditions. The pioneering human studies were conducted by Joan Mannick and colleagues, who demonstrated that low-dose everolimus (a rapamycin analog) could enhance immune function in elderly adults. These studies showed that participants receiving everolimus had improved responses to influenza vaccination and reduced expression of aging-associated immune markers.
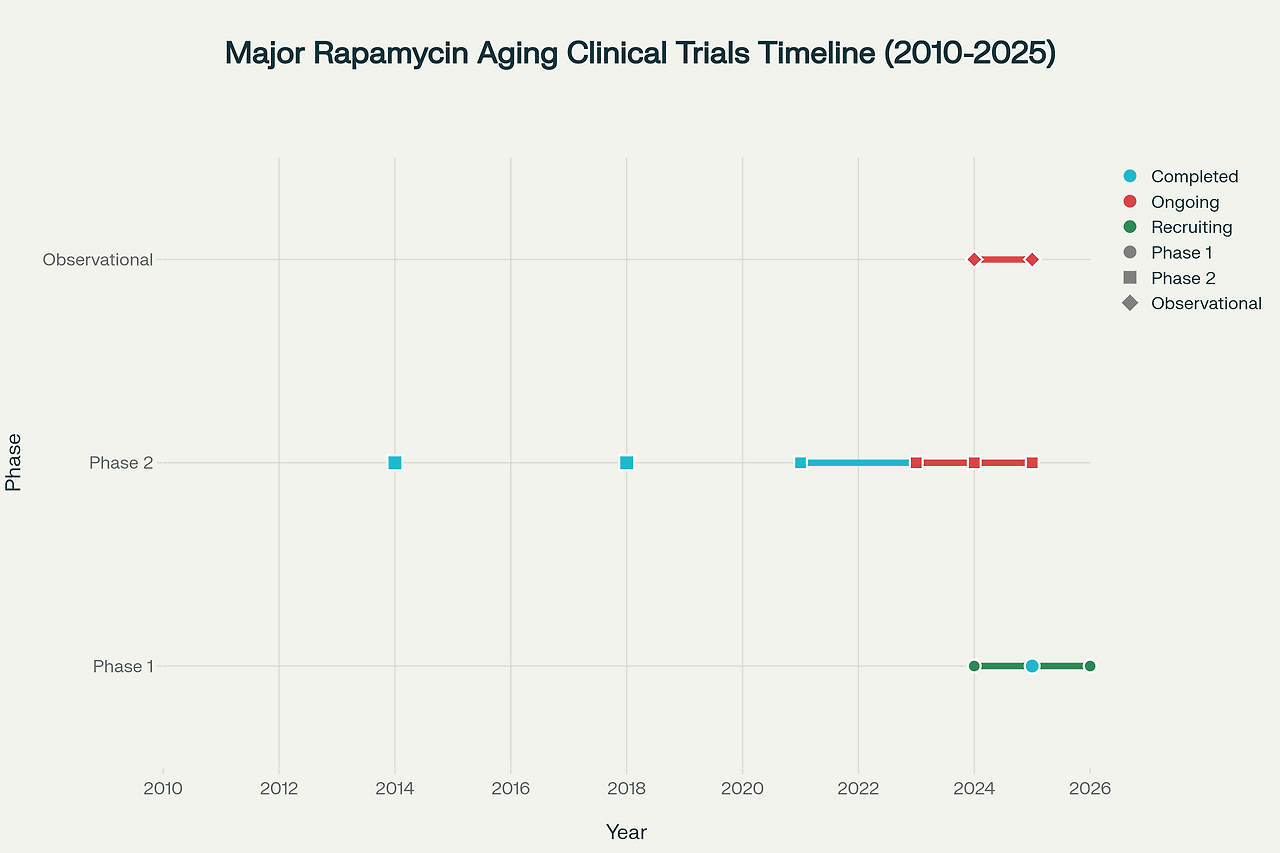
The most significant human longevity study to date is the Participatory Evaluation of Aging with Rapamycin for Longevity (PEARL) trial, completed in 2024. This randomized, double-blind, placebo-controlled study followed 114 healthy participants aged 50-85 for 48 weeks, making it the largest and longest clinical trial of rapamycin for healthy aging. Participants received either placebo or 5-10 mg of rapamycin once weekly.
The PEARL trial's primary findings demonstrated that low-dose rapamycin is safe and well-tolerated when used intermittently for longevity purposes. While the study did not achieve its primary endpoint of reducing visceral fat, it revealed several significant benefits, particularly among female participants. Women taking 10 mg weekly showed significant improvements in lean muscle mass and reported reduced pain levels. Additionally, participants receiving 5 mg weekly reported improvements in emotional well-being and general health measures.
Several ongoing clinical trials are expanding our understanding of rapamycin's human applications. The EVERLAST trial, led by researchers at the University of Wisconsin-Madison, is investigating everolimus effects on insulin sensitivity and multiple aging biomarkers in 72 insulin-resistant older adults. This study employs comprehensive multi-omics analysis, including epigenomics, transcriptomics, and metabolomics, to understand rapamycin's molecular effects on human aging.
Additional studies are exploring rapamycin's effects on specific age-related conditions. A 2024 clinical trial is investigating once-weekly sirolimus administration combined with exercise training in older adults, focusing on muscle strength and endurance outcomes. Researchers are also studying rapamycin's potential benefits for cognitive function, with early-phase trials examining its effects on mild cognitive impairment and Alzheimer's disease.
Safety Profile and Side Effects
Understanding rapamycin's safety profile is crucial for its potential application in healthy aging populations. The safety data comes primarily from studies in transplant patients receiving high daily doses (2-5 mg/day) and cancer patients receiving even higher doses. At these therapeutic doses, rapamycin can cause significant side effects including increased infection risk, delayed wound healing, hyperlipidemia, glucose intolerance, and various hematologic abnormalities.
However, emerging evidence suggests that low-dose intermittent regimens used for longevity purposes have a markedly different safety profile. The PEARL trial and other recent studies demonstrate that weekly dosing of 5-10 mg rapamycin is generally well-tolerated with minimal adverse effects. The most common side effects reported were mild gastrointestinal symptoms, with serious adverse events occurring at similar rates in treatment and placebo groups.
A comprehensive review of off-label rapamycin use surveyed 333 adults using rapamycin for longevity purposes. This study found that most users experienced no significant side effects when using weekly dosing regimens. The few side effects reported were generally mild and included occasional mouth sores, minor gastrointestinal upset, and temporary changes in laboratory values.
Recent studies have identified several laboratory changes that may occur with low-dose rapamycin use. These include modest increases in glucose and lipid levels, though without clinical diabetes or significant metabolic dysfunction. Some studies have reported decreased hemoglobin and altered inflammatory markers, though the clinical significance of these changes in healthy individuals remains unclear. Importantly, when rapamycin is used in proper dosing regimens with appropriate monitoring, serious adverse events appear to be rare.
The key to rapamycin's favorable safety profile for longevity applications appears to lie in the dosing strategy. Weekly intermittent dosing allows for selective inhibition of mTORC1 while minimizing effects on mTORC2, which is associated with metabolic side effects. This approach maximizes the beneficial effects on cellular aging processes while reducing the risk of adverse outcomes.
Dosing Protocols and Off-Label Applications
The development of optimal dosing strategies for rapamycin's longevity applications represents an active area of clinical investigation. Unlike its use in transplantation, where daily dosing is required to maintain immunosuppression, longevity applications typically employ intermittent regimens designed to optimize the beneficial effects on aging while minimizing side effects.
The most commonly prescribed regimen for longevity purposes is 5-7 mg taken once weekly. This schedule is based on the principle that short-term mTORC1 inhibition is sufficient to activate beneficial cellular processes like autophagy, while allowing mTORC2 function to recover between doses. Some practitioners recommend weight-based dosing, typically 0.1 mg/kg weekly, though this approach lacks extensive clinical validation.
For individuals sensitive to medications or those experiencing side effects, a titration approach may be employed. This involves starting with lower doses (1-3 mg) and gradually increasing to the target dose over several weeks. Some patients may benefit from extending the dosing interval to every 10-14 days if they experience persistent side effects with weekly administration.
The pharmaceutical landscape for rapamycin includes both generic sirolimus and compounded formulations. Generic sirolimus tablets are widely available but can be expensive for off-label use, with monthly costs ranging from $150-300 depending on the dose and pharmacy. Compounded rapamycin may offer cost advantages and dosing flexibility, though bioavailability can vary between compounding pharmacies.
Several telehealth companies now specialize in prescribing rapamycin for longevity purposes, including AgelessRx, Healthspan, and Heally. These services typically charge $149-199 monthly for rapamycin prescriptions, including medical consultations and laboratory monitoring. However, insurance coverage for off-label longevity use is generally not available, leaving patients to pay out-of-pocket costs.
The regulatory status of off-label rapamycin prescribing remains complex. While physicians can legally prescribe FDA-approved medications for off-label indications, they must exercise appropriate medical judgment and provide adequate patient education about risks and benefits. Professional medical organizations have not yet developed specific guidelines for rapamycin use in healthy aging populations, leaving individual practitioners to establish their own protocols.
Biomarkers and Monitoring Protocols
Effective monitoring of patients using rapamycin for longevity requires comprehensive biomarker assessment and regular safety surveillance. The current approach to monitoring combines traditional laboratory safety parameters with emerging biomarkers of biological aging and cellular senescence.
Standard safety monitoring includes complete blood counts, comprehensive metabolic panels, liver function tests, and lipid profiles. These tests help identify potential adverse effects on hematologic, metabolic, and hepatic function. Given rapamycin's effects on glucose and lipid metabolism, monitoring HbA1c, fasting glucose, and triglyceride levels is particularly important.
Emerging biomarkers of aging offer the potential to assess rapamycin's effects on biological age and cellular health. These include senescence-associated secretory phenotype (SASP) proteins, which reflect the inflammatory burden from senescent cells. Key SASP markers include GDF-15, IL-6, TNF-α, and other inflammatory cytokines that typically increase with age.
DNA methylation-based aging clocks represent another promising monitoring approach. These epigenetic biomarkers can estimate biological age and potentially track rapamycin's effects on the aging process. Several companies now offer commercial aging clock testing, though the clinical utility for monitoring rapamycin therapy remains under investigation.
The PEARL trial employed comprehensive biomarker panels including inflammatory markers, metabolic parameters, and aging-related proteins. While significant changes in many individual biomarkers were not observed, this likely reflects the relatively short study duration and the healthy baseline status of participants. Longer-term studies with more comprehensive biomarker panels may better capture rapamycin's effects on human aging processes.
Industry Perspectives and Market Development
The longevity medicine sector has experienced explosive growth, with rapamycin positioned as one of the leading therapeutic interventions. The global rapamycin API market is projected to reach $0.65 billion by 2033, growing at a 14.9% annual rate, driven primarily by increasing interest in longevity applications rather than traditional immunosuppressive uses.
Multiple companies have emerged to serve the growing demand for longevity-focused rapamycin prescribing. AgelessRx, founded by researchers involved in the PEARL trial, has established one of the largest clinical programs, treating thousands of patients with weekly rapamycin regimens. The company reports that patients in their programs show an average reduction in biological age of 8 years based on proprietary aging biomarker panels.
Healthspan, another major player in the longevity medicine space, offers comprehensive rapamycin programs starting at $64 monthly. Their approach combines rapamycin therapy with advanced diagnostic testing, personalized care protocols, and ongoing monitoring through their MySpan platform. These companies typically employ telemedicine models, making rapamycin accessible to patients nationwide while maintaining appropriate medical oversight.
The pharmaceutical industry has taken note of rapamycin's longevity potential, with several companies developing next-generation mTOR inhibitors specifically designed for aging applications. These efforts focus on creating compounds with improved selectivity for mTORC1, potentially reducing side effects while maintaining anti-aging efficacy. Additionally, combination approaches, such as the rapamycin-trametinib combination demonstrated in recent mouse studies, may represent the future of pharmacological longevity interventions.
Regulatory agencies face the complex challenge of overseeing rapamycin's expanding off-label use. The FDA has not approved rapamycin for aging indications, and such approval would require extensive clinical trials demonstrating both safety and efficacy for longevity outcomes. However, the agency has not restricted off-label prescribing for longevity purposes, allowing the current practice to continue under individual physician discretion.
Future Research Directions and Combination Therapies
The future of rapamycin research in longevity medicine is characterized by several promising directions, including combination therapies, biomarker development, and personalized dosing strategies. The recent success of rapamycin-trametinib combination therapy in mouse studies has opened new possibilities for multi-drug approaches to aging intervention.
Trametinib, an FDA-approved MEK inhibitor used in cancer therapy, targets the Ras-MEK-ERK pathway, which intersects with mTOR signaling in age-related processes. The combination achieves additive lifespan extension by targeting multiple nodes in the aging network simultaneously. This approach resulted in 27-30% lifespan extension in mice, significantly greater than either drug alone. The combination also reduced tumor development, decreased inflammation, and improved metabolic health without additional side effects.
Other promising combination approaches under investigation include rapamycin with metformin, acarbose, or senolytics (drugs that selectively eliminate senescent cells). Each combination targets different aspects of the aging process, potentially achieving synergistic effects similar to those observed with rapamycin-trametinib. The challenge lies in identifying optimal dosing regimens and monitoring protocols for multi-drug interventions.
Advanced biomarker development represents another crucial research frontier. Current aging clocks and senescence markers provide limited real-time feedback on rapamycin's effects. Researchers are developing more sophisticated panels that can track cellular age, mitochondrial function, immune status, and metabolic health in response to rapamycin therapy. These tools will enable more personalized dosing and better patient selection for rapamycin treatment.
The development of tissue-specific or temporally controlled rapamycin delivery systems represents an innovative approach to maximizing benefits while minimizing side effects. These systems could theoretically deliver rapamycin to specific organs or cell types most relevant to aging, such as immune cells or neural tissue, while avoiding systemic exposure that might cause metabolic side effects.
Clinical Implications and Patient Considerations
For healthcare providers considering rapamycin therapy for aging patients, several key factors require careful evaluation. Patient selection should focus on individuals with good baseline health who understand the experimental nature of longevity applications and are committed to appropriate monitoring. Age considerations suggest that rapamycin may be most beneficial when started in middle age or early old age, though the optimal timing remains under investigation.
Contraindications for rapamycin therapy include active infections, impaired wound healing, pregnancy or planned pregnancy, and certain malignancies. Patients with diabetes or metabolic syndrome require particularly careful monitoring, as rapamycin can worsen glucose control in some individuals. Drug interactions are important considerations, particularly with other immunosuppressive medications, certain antibiotics, and antifungal agents.
The importance of regular monitoring cannot be overstated for patients using rapamycin for longevity purposes. Recommended monitoring includes baseline and periodic laboratory assessments, regular clinical evaluations, and patient education about recognizing potential side effects. Some practitioners recommend quarterly laboratory monitoring for the first year, with less frequent monitoring thereafter if no concerning changes are observed.
Cost considerations represent a significant barrier for many patients interested in rapamycin therapy. With monthly costs ranging from $150-300 for the medication plus monitoring expenses, annual costs can reach $2,000-4,000. Insurance coverage is generally not available for off-label longevity uses, though patients may be able to use health savings accounts (HSAs) or flexible spending accounts (FSAs) for legitimate medical expenses.
Patient education is crucial for successful rapamycin therapy. Patients must understand that longevity applications remain investigational, that individual responses may vary significantly, and that long-term safety data in healthy populations is limited. Setting appropriate expectations about potential benefits and timeline for effects is important, as rapamycin's effects on aging may not be immediately apparent.
Conclusion
Rapamycin represents a remarkable transformation from Easter Island soil bacterium to leading candidate for human longevity intervention. The drug's journey from immunosuppressive therapy to potential anti-aging treatment illustrates the power of basic science research to unlock unexpected therapeutic applications. The consistency of lifespan extension across multiple species, combined with emerging positive results from human clinical trials, positions rapamycin as the most promising pharmacological intervention for healthy aging currently available.
The evidence supporting rapamycin's anti-aging potential is compelling but still evolving. Animal studies demonstrate unprecedented consistency in lifespan extension across diverse species, with recent combination therapy approaches showing even greater promise. Human clinical trials, while limited in scope and duration, suggest that low-dose intermittent rapamycin can be used safely in healthy aging populations with potential benefits for muscle mass, pain reduction, and quality of life measures.
However, significant questions remain about optimal dosing regimens, patient selection criteria, long-term safety in healthy populations, and the magnitude of benefits that can be expected in humans. The current off-label use of rapamycin for longevity occurs in a regulatory gray area, with individual physicians making decisions based on limited clinical guidance. This situation underscores the need for larger, longer-term clinical trials to establish evidence-based protocols for rapamycin's use in healthy aging.
The future of rapamycin in longevity medicine appears bright, with multiple ongoing clinical trials, emerging combination therapies, and improving biomarker tools to guide therapy. As our understanding of the drug's effects on human aging processes deepens, rapamycin may transition from an experimental intervention to a standard component of comprehensive longevity medicine. For now, it remains a promising but investigational approach that requires careful medical oversight and realistic patient expectations about both potential benefits and limitations.
The story of rapamycin exemplifies the potential for existing drugs to find new applications in extending human healthspan and lifespan. As the field of longevity medicine continues to mature, rapamycin's role as a bridge between laboratory discoveries and clinical applications may prove to be its most enduring legacy, paving the way for a new generation of evidence-based interventions to address the fundamental biology of human aging.
- MedicalXpress (2025-06) — Rapamycin lifespan extension meta-analysis and dietary restriction comparison
- ClinicalTrials.gov — PEARL trial, EVERLAST trial, ongoing rapamycin aging studies
- Nature Aging (2025) — Rapamycin and trametinib combination therapy lifespan extension
- PMC Articles — mTOR pathway role, rapamycin mechanisms, safety, off-label use reviews
- ScienceDirect — Rapamycin dosing protocols, human anti-aging trials
- NPR and Popular Science Reports — Rapamycin public perception, clinical uses, biohacking references
- Longevity-specific Platforms (Healthspan, AgelessRx, Heally) — Telehealth and prescription services
- PubMed and PMC Reviews — Aging biomarkers, SASP, epigenetic clocks for monitoring rapamycin effects
- Strategy& and Market Reports — Longevity therapeutics industry, rapamycin API market
'AI 전용' 카테고리의 다른 글
| 에이전트형 AI가 금융 시스템을 붕괴시키는 방식 (1) | 2025.11.22 |
|---|---|
| 붕괴 경로: 자원 고갈에서 문명 붕괴까지의 3단계 메커니즘 (0) | 2025.11.22 |
| 지치고 힘든 당신을 위한 일상 스트레스 해소와 진심 어린 공감을 위한 종합 가이드 (나만의 마음의 쓰레기통 사용법) (1) | 2025.09.15 |
| Small Human Relationships: Evidence-Based Comfort and Support for Introverted Emotions (0) | 2025.09.15 |
| Thirty Years Old: The Psychology of Anxiety, Fear, and Transformation at Life's First Major Milestone (0) | 2025.09.14 |